Why Isn't There a Herpes Vaccine Yet?

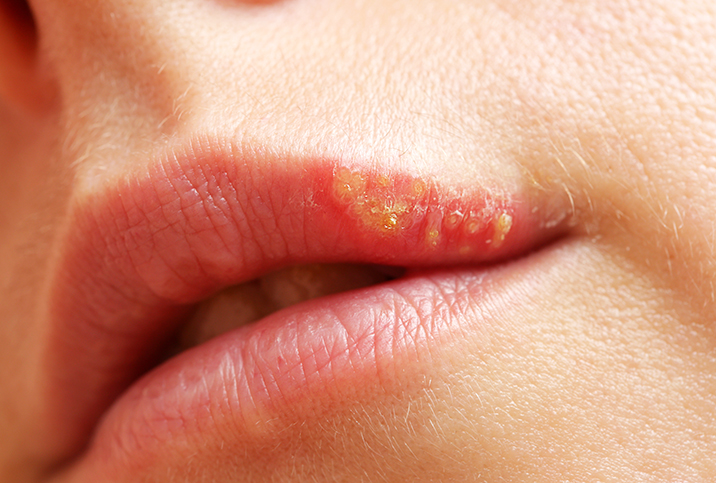
A viral pandemic is raging across the globe. More than a billion people have it, and if you've got it, you can pass it on through close contact with loved ones. It's called HSV, but you might know it as herpes. Almost half of the American population has HSV-1, oral herpes, the kind that gives you cold sores around your mouth. Less visibly, 1 in every 10 people has genital herpes, or HSV-2—painful, itchy little blisters that periodically flare up in the nether regions. It can be painful, itchy and the subject of some awkward conversations with new sexual partners.
Currently, there isn't a cure. You can manage the symptoms, but once you've got herpes, it's with you for life. Given how many people suffer from this nasty condition, why don't we have a vaccine yet? And will what we've learned from COVID-19 help us get to a herpes vaccine faster?
Tons of people live with herpes. How bad can it be?
If you have oral or genital herpes, it likely flares up every now and then, causing nasty sores around the genitals or mouth, which can be treated with antiviral medication, and then it goes away. The symptoms are nasty and distressing, and in 2008, cost the world $541 million U.S. But there are also a few situations in which herpes can be life-threatening.
Most notably, having genital herpes increases your chance of contracting and transmitting HIV, even when you're asymptomatic. This is a serious problem in regions where both genital herpes and HIV are widespread, such as Sub-Saharan Africa. The World Health Organization is backing efforts to develop a herpes vaccine, in part because it should help reduce the worldwide burden of HIV.
Another major threat from genital herpes is neonatal herpes, where a herpes-positive mother can pass that infection on to her newborn child. If left untreated, neonatal herpes can be fatal, but treatments are usually effective in keeping the newborn safe.
A super-smart virus
Scientists have so far been unable to create a herpes vaccine because herpes is the overachiever of biological hide-and-seek. "The immune system has a hard time finding the virus when it's first introduced into the body," said Professor Keith Jerome, whose Seattle lab is working on a gene therapy treatment for herpes. "Very quickly, the virus gets into nerves where the immune system can't find it." Oral herpes hides out near the brain, while genital herpes makes itself at home in the dorsal root ganglion, close to the spine. Occasionally, it hitches a ride down nerves to create symptoms. "It's a very difficult virus for vaccination because the immune system has to react so quickly before the virus gets to the nerves," Jerome said.
What about a COVID-style mRNA vaccine?
In 2020, several vaccine manufacturers successfully created a COVID-19 vaccine in record time, with many using mRNA technologies. Most vaccines use a weakened type of the virus to teach our immune systems how to mount a response to the full-strength virus if we come into contact with it. Instead, mRNA vaccines don't use the original virus at all. They teach our bodies to manufacture a harmless "spike protein," which to our immune systems looks like the COVID-19 virus. That spike protein triggers an immune response, which produces antibodies, protecting us from becoming infected with the real virus.
mRNA vaccine technology was developed by scientists at the University of Pennsylvania in the decades before COVID struck. While no mRNA vaccines had been approved, one flu vaccine had been tested, and another University of Pennsylvania team had developed an mRNA vaccine for—you guessed it—herpes.
In 2019, a team in Professor Harvey Freidman's lab successfully inoculated 63 out of 64 guinea pigs against genital herpes, and 8 out of 10 mice, using an mRNA herpes vaccine. They've had to overcome a few tricky obstacles.
"For herpes, you need immunity for more than just a few years," said Sita Awasthi, Ph.D., a senior researcher in the team creating the herpes vaccine. You need to be protected for your entire sexually active lifetime. While it's yet to be tested in humans, it's likely that herpes booster shots won't be needed. "The problem with COVID is the virus keeps changing or mutating, making multiple variants," Awasthi said. "Herpes is a DNA virus, so it does not vary from person to person or over time. It's possible that one may have a long-term immunity." But to know that, researchers need to test the vaccine over a long period in humans, something that's yet to come.
For the vaccine to progress to human trials, a lot needs to happen. Right now, the vaccine is being developed in a lab, which is fine for initial tests, but a lot more safety checks need to happen before it's tested on humans. Plans for these trials are in progress.
What about a cure?
Scientists at the Fred Hutchinson Cancer Research Center are taking a different tack by trying to cure HSV-1 herpes using gene editing, a technique that uses molecular scissors that enter cells to look for certain DNA patterns that only exist in the herpes virus. Once the pattern is found, the gene-editing treatment snips the virus in half so that it's no longer able to cause symptoms or be transmitted to another person.
They've tested this approach in animals, and it eliminated 90 percent of the virus—enough, they believe, to stop it from causing symptoms, and enough to stop the animal from transmitting it to others. Their research was published in August 2020. Now, the researchers are attempting the same thing with HSV-2, the type of the virus that usually causes genital herpes. If successful, they expect it'll take about three years before the treatment is ready to be tested in humans.
For both a vaccine and a cure, the future looks positive. Awasthi and her team are pushing on, motivated by the huge scale of the problem: "It's important for me to help people," she said, "because there are certainly a lot of people who are suffering."