The FDA Greenlights a Plan B Label Change
New emergency contraception packaging may soon be on its way to a pharmacy near you.
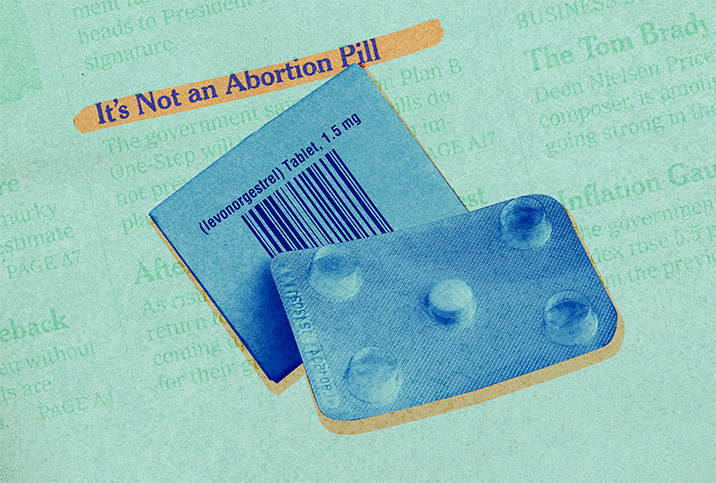
The U.S. Food and Drug Administration (FDA) announced its approval of updated label changes for the over-the-counter emergency contraception pill Plan B One-Step, commonly referred to as the "morning after pill," in a 27-page memo released Dec. 23.
The new FDA label specifies Plan B is not an abortion medication but rather a form of emergency contraception. Abortion medication, such as misoprostol, is used to terminate an existing pregnancy, whereas emergency contraception is effective only in preventing pregnancy when used before fertilization occurs.
The popular emergency contraception brand Plan B One-Step, produced by Foundation Consumer Healthcare, requested the FDA update. Plan B is most effective at preventing pregnancy when taken up to 72 hours after unprotected sex.
Why change the label?
Citing new and existing research, Foundation Consumer Healthcare asserted "updates to the labeling are needed to make the labeling more accurate, to reduce consumer confusion, and potentially to reduce barriers to use of the legally marketed approved product," according to the FDA memo.
"As explained in this review, LNG-EC [levonorgestrel-only emergency contraception] prevents pregnancy—it does not terminate pregnancy," the memo stated. "For people who are capable of becoming pregnant, LNG-EC is effective in preventing pregnancy when taken within three days of unprotected intercourse and before ovulation. LNG-EC is not effective if pregnancy has already occurred."
The FDA's announcement marks the first update to Plan B One-Step packaging since it was first approved for over-the-counter release in 2006.
How does Plan B work?
The Plan B pill, when taken as directed, releases a large dose of the synthetic hormone levonorgestrel, effectively stopping or delaying the release of an unfertilized egg from the ovary. Research shows levonorgestrel can inhibit or delay ovulation, but it does not have a direct effect on implantation or fertilization.
Previously, Plan B's packaging indicated the medication could possibly prevent a fertilized egg from attaching to the uterine wall, however, research does not support that initial claim.
A review of evidence by the American College of Obstetricians and Gynecologists affirmed emergency contraception medication is "unlikely to prevent implantation of a fertilized egg."
A year of change
The FDA's label change comes days before the close of a particularly tumultuous year for reproductive rights.
In the six months since the Supreme Court overturned Roe v. Wade, legislation that protected a woman's constitutional right to abortion, 12 states have criminalized abortion.
On the heels of the landmark court ruling, abortion rights advocates warned contraception, and emergency contraception, may be next in the American battle for reproductive rights as some states have made it clear contraception is next in line for discussion.
In his June 2022 opinion, Supreme Court Justice Clarence Thomas said the court "should reconsider" past contraception rulings.
Thomas' opinion on contraception may be an unpopular one with the vast majority of American women. Approximately 90 percent of women ages 18 to 64 have used contraception at some point in their reproductive years, according to a 2022 survey by the Kaiser Family Foundation.
Nearly a quarter of reproductive-aged women have used some form of emergency contraception in their lifetime, according to a 2021 study by the Centers for Disease Control and Prevention (CDC).
As of Dec. 1, 2022, nine states have restricted access to emergency contraception medication, according to the Guttmacher Institute, a research and policy nonprofit organization.
It is illegal to have an abortion, whether surgically or medication-induced, in the following 12 states: Idaho, Wisconsin, Oklahoma, Arkansas, South Dakota, Missouri, Texas, Louisiana, Mississippi, Tennessee, Kentucky and West Virginia.