FDA Approves Dual-Target Treatment for Type 2 Diabetes
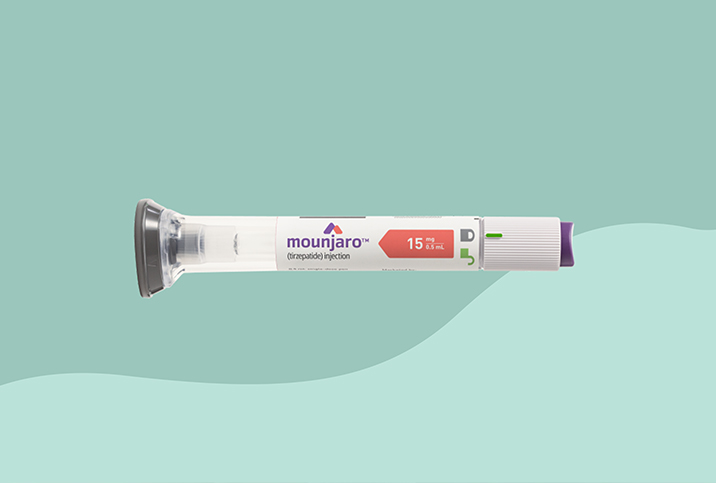
The Food and Drug Administration (FDA) officially approved Mounjaro (tirzepatide) as a receptor-agonist treatment for GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 (glucagon-like peptide-1) in adults with type 2 diabetes.
The once-weekly injection is intended for use alongside diet and exercise to improve glycemic control in adult patients diagnosed with the disease.
"Given the challenges many patients experience in achieving their target blood sugar goals, [the] approval of Mounjaro is an important advance in the treatment of type 2 diabetes," Patrick Archdeacon, M.D., an associate director of the Division of Diabetes, Lipid Disorders and Obesity in the FDA's Center for Drug Evaluation and Research, said in a press release.
The most common form of diabetes, type 2 diabetes is a chronic and progressive condition in which the body does not make or use insulin normally, leading to high levels of glucose in the blood, according to the FDA. High levels of blood glucose can lead to nerve and vascular damage, both of which can affect men's ability to achieve an erection.
Despite the many medications available to treat diabetes, some patients still struggle to achieve and maintain the recommended blood sugar levels.
How tirzepatide can help adults with type 2 diabetes
GLP-1 and GIP are hormones involved in controlling a person's blood sugar. Medications such as metformin, insulin, sulfonylureas and meglitinides are prescribed to help control either GLP-1 or GIP. Mounjaro activates both the GLP-1 and GIP receptors simultaneously to help improve blood sugar control.
The FDA press release stated, "Mounjaro was effective at improving blood sugar and was more effective than the other diabetes therapies with which it was compared in clinical studies."
The study
The newly approved medication comes in three different doses administered via once-weekly injections: 5 milligrams, 10 mg and 15 mg. These doses were evaluated in five clinical trials, either as a standalone therapy or an add-on to other diabetes medications.
Mounjaro, developed by Eli Lilly and Co., was compared with a placebo, a GLP-1 receptor agonist (semaglutide) and two long-acting insulin analogs, which are medications designed to mimic the body's natural pattern of insulin release.
On average, patients who received the 15-mg dose lowered their hemoglobin A1C (HbA1C) level, which measures blood sugar control, 1.6 percent more than patients on a placebo. This same dosage lowered patients' HbA1C levels 0.5 percent more than semaglutide, 0.9 percent more than insulin degludec, and 1 percent more than insulin glargine.
Researchers also discovered the use of Mounjaro helped patients lose weight by an average of 15 pounds more than a placebo alone, and 23 pounds more than a placebo when each was used with insulin.